UIC physicists find hidden flaw in century-old, common chemical extraction technique
For nearly a century, a technique called liquid-liquid extraction has been standard practice in science and industry. It’s used to purify rare earth metals, treat nuclear waste and make perfumes and other consumer products.
But a team of UIC researchers have discovered a previously undetected flaw in the process. Correcting it could point the way to more efficient extraction with the widely used technique.
The discovery was made by the research group of Mark Schlossman, physics professor at UIC and an expert on the phenomena that occurs at liquid-liquid interfaces. Their research, published recently in Proceedings of the National Academy of Sciences, describes a previously unobserved form of precipitation that decreases the yield of the desired extract.
In liquid-liquid extraction, two fluids that can’t mix are combined — typically water and an organic solvent. Then an impure material, such as ore containing rare earth metals used in high-tech products, is added. Through a process called ion transport, the desired metal moves from the water (the aqueous phase) into the solvent (the organic phase), where it can be captured and further purified.
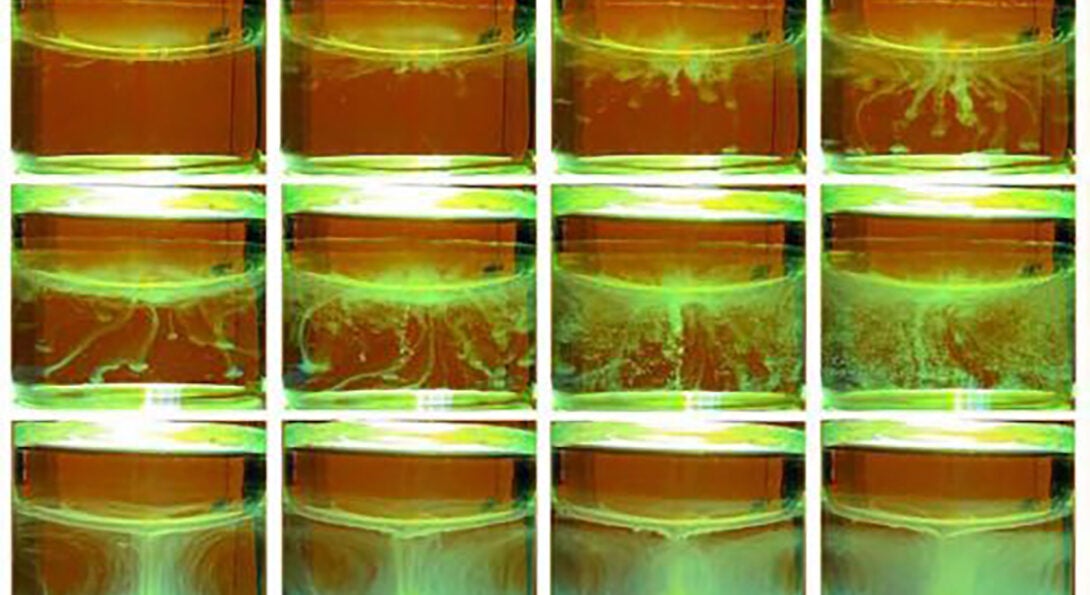
An unwelcome byproduct of the extraction is precipitate, a solid crust colloquially called crud. It forms at the border between the water and solvent and reduces the amount of material harvested.
But, by accident, former UIC postdoctoral researcher Pan Sun discovered a second source of precipitate on the water side in a liquid-liquid sample. This second source of precipitate and its dynamics had not been previously observed, according to the researchers.
“A lot of things are happening in the aqueous phase that people, including us, were just not expecting to see,” Schlossman said. “This pathway is actually diverting things in the wrong direction, and it makes the process slower.”
Further experiments showed that tweaking the conditions of the reaction, such as the pH or metal concentration, increased or reduced how much precipitate formed in the water. These insights could help refine industrial extraction processes to yield less precipitate and more of the intended product.
“Based on what we observed, I think we can build a new kinetics model for people to predict their outcomes better,” said Sun, now a researcher at Oak Ridge National Laboratory. “You can take advantage of these conditions to get better efficiency or selectivity for different metal ions.”
The research was funded by grants from the U.S. Department of Energy. Some of the experiments were done at the National Science Foundation-funded ChemMatCARS, a research facility at the Advanced Photon Source of Argonne National Laboratory run by a collaboration between UIC and University of Chicago.